SESSION 1: CATALYSIS AND ADSORPTION IN MOFS
Unraveling the Thermodynamic Conditions for Negative Gas Adsorption in Soft Porous Crystals
Center for Molecular Modeling, Ghent University, Technologiepark 46, Zwijnaarde 9052, Belgium
Keywords: soft porous crystals, negative gas adsorption, free energy, mechanical pressure, adsorption isotherm
Metal-organic frameworks (MOFs) are among the most intriguing materials of current science. Even though MOFs are crystalline, some have shown flexible behavior in the sense that they are capable of transforming between various phases accompanied by substantial changes in the unit cell volume. Kitagawa coined the term “soft porous crystals” (SPC) for materials that show a bistable or multistable behavior with long–range structural order and permanent porosity [1]. These SPCs are known for their intriguing properties and various counterintuitive phenomena such as negative linear compression, negative thermal expansion and negative gas adsorption (NGA). The latter was illustrated by the adsorption of methane in DUT-49 for which experimentally a drop in the amount of adsorbed methane was observed under increasing vapor pressure [2]. Recently, we proposed a generalized thermodynamic approach to classify SPCs into various types of flexibility upon exposure to mechanical pressure based on the Helmholtz free energy of the empty materials [3]. In the current work, we construct the Helmholtz free energy by means of a semi-analytical thermodynamic model[4] and build further upon this classification to investigate whether there is a correlation between pressure-induced breathing of the empty framework and adsorption-induced breathing at fixed mechanical pressure. As such we aim to determine the conditions required for NGA and investigate the correlation with pressure-induced breathing.
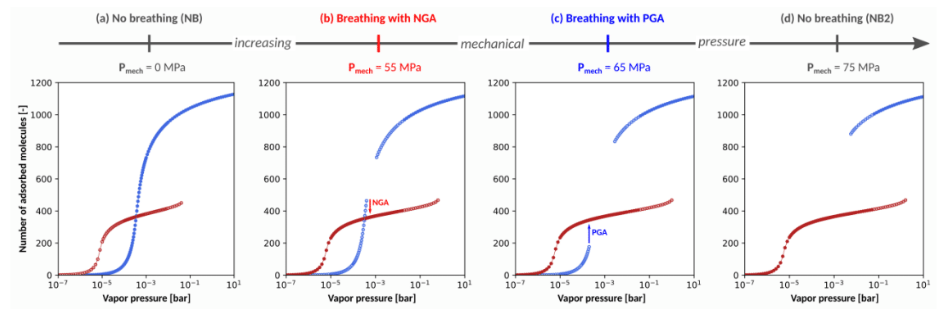
Figure 1 illustrates the adsorption isotherm for methane in a hypothetical non-breathing MOF (i.e., non-breathing in absence of mechanical pressure) for increasing values of an applied mechanical pressure, showing that increasing the mechanical pressure can induce NGA. Therefore, even though NGA was experimentally observed for methane adsorption in DUT-49, we herein discovered that it could be a more generally applicable phenomenon under influence of guest adsorption and external mechanical pressure, evenindicating that NGA might become tuneable. Although it is very difficult to perform the required experiments to test such statements with the currently available experimental setups, the present results still allows us to gain crucial insight into the NGA phenomenon. Furthermore, such meticulous control of multiple triggers for NGA can open the way to new applications such as tuneable gas detection and pressure amplification.
Figure 1: Illustration of the impact of applying mechanical pressure onthe adsorption isotherm of an initially non-breathing MOF. The blue and red dots represent the number of molecules adsorbed in the open pore and contracted pore state respectively.
[1] Horike, S., Shimomura, S. and Kitagawa, S.,Nat. Chem. 1, 695, 2009
[2] Krause, S. et al., Nature 532, 348, 2016
[3] Vanduyfhuys, L. et al., Nat. Commun. 9, 204, 2018
[4] Vanduyfhuys, L. et al., Mol. Simulat. 41, 1311–1328, 2015